
Study Design:
A phase 3, multicenter, multi-country, open-label, randomised, active-controlled clinical trial.20
Primary Endpoint:
Mean change in Hb levels from baseline (BL) to evaluation (Week 16 to Week 24) period in modified intent-to-treat (mITT) population.20
N= 588 patients with (stages 3-5 CKD, aged 18-80 years old)*
*with a haemoglobin (Hb) level between 7-10 g/dL were randomised to receive either desidustat oral tablet or darbepoetin alfa injection for 24 week.20
Dose: Desisutat 100 mg 3 times a week (2 days apart and not 4 days apart)20
Duration: 24 weeks
Key Results: Primary endpoint analysis20
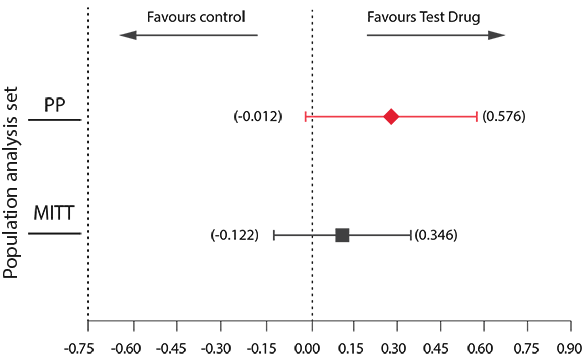
Plot of 95% CI of difference (%) in Hb between Desidustat and Darbepoetin
Secondary Endpoint Analysis:20
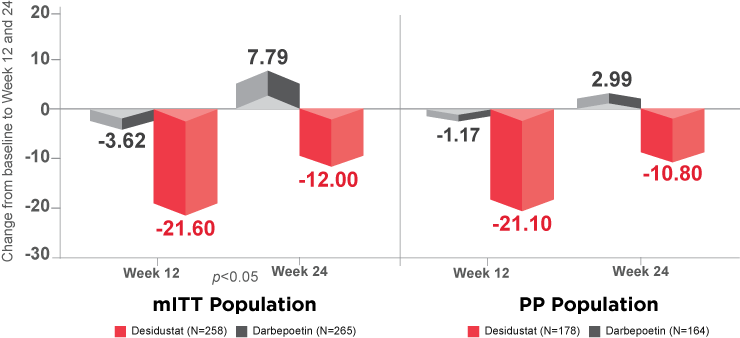
as represented by secondary end points, i.e., for time to achieve target Hb (p=0.2985), and for percentage time spent in target Hb range (p=0.1113).20
(p = 0.0268) in low density lipoprotein (LDL)-cholesterol at Week 24 compared to baseline.20
Safety Results:
The number of patients experiencing AEs during the trial do not significantly differ in the treatment and placebo arms.20
Conclusion:
Desidustat was found to be non-inferior compared to darbepoetin in the treatment of anaemia in patients with CKD who were not on dialysis. The safety of desidustat is comparable to darbepoetin.20
REFERENCES:
Study Design:
A phase 3, multicenter, open-label, randomised, active-controlled study21.
Primary Endpoint:
Primary efficacy endpoint was evaluation of difference of mean change of Hb from baseline (BL) to evaluation period (Week 16-24)21.
N=392 CKD patients (stage V) on dialysis*
*Subjects were randomised to recieve either Desidustat oral tablet or Epoetin injections for 24 weeks depending on previous ESA dose21.
Dose: Desidustat (100, 125, 150 mg) three times a week (2 days apart and not 4 days apart)21.
Duration: 24 weeks
Key Results: Primary endpoint analysis21
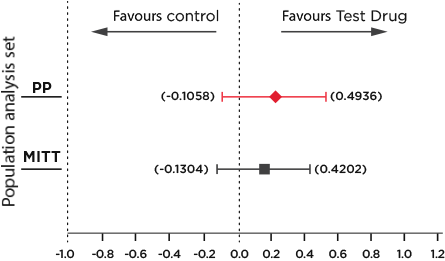
The change from baseline Hb (16-24 week)21:
Desidustat: 0.95 g/dl
Epoetin alfa: 0.80 g/dl
The lower limit of the 95% CI was above the predefined non-inferiority margin of –1.0 g/dL
Secondary Endpoint Analysis21:
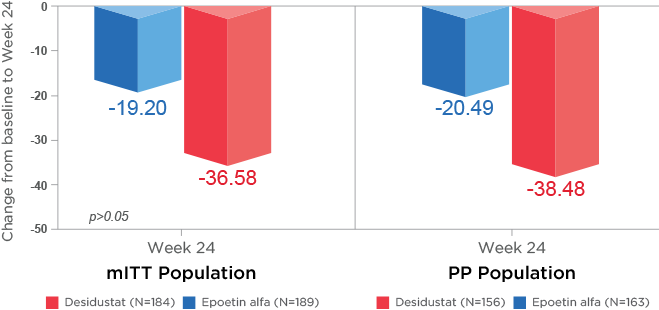
as represented by secondary end points, i.e., for time to achieve target Hb (p=0.041), and percentage of responders (p=0.038)21.
(p<0.0001) at Week 12 when compared to baseline21.
Safety Results:
The number of patients experiencing AEs during the trial do not differ in both the arms21.
Conclusion:
Desidustat was found to be non-inferior compared to epoetin in the treatment of anaemia in patients with CKD who were on dialysis. The safety of desidustat is comparable to epoetin21.
REFERENCES: