
Desidustat (INN, also known as ZYAN1) is a drug for the treatment of anaemia of chronic kidney disease. This drug with the brand name Oxemia™ is discovered and developed by Zydus Life Sciences.13
 Desidustat is a HIF prolyl-hydroxylase inhibitor.13
Desidustat is a HIF prolyl-hydroxylase inhibitor.13
Hypoxia Inducible Factor (HIF) - What is it?
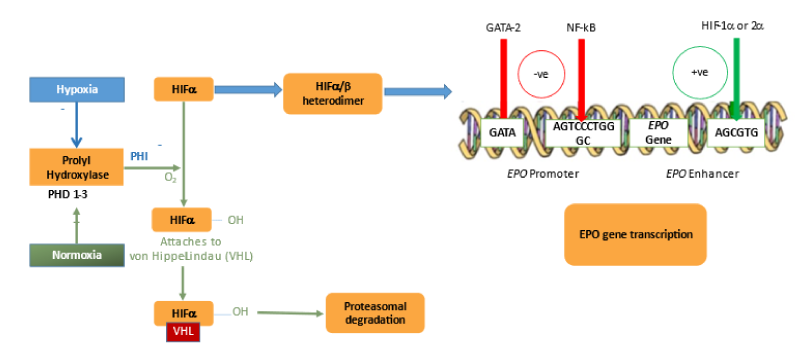
HIF, a critical intracellular modulator of hypoxia-induced reactions and first discovered in the 1950s, comprises two different subunits, alfa and beta chains. The alfa chains are all oxygen sensitive and have 3 main forms (HIF-1a, HIF-2a and HIF-3a), whereas the beta chains are constitutively expressed (also known as the aryl hydrocarbon nuclear translocator (ARNT). Both HIF-1a and HIF-2a together with ARNT form HIF-1 and HIF-2 transcription factors, respectively. HIF-a subunits are immediately degraded under normoxic conditions. HIF2 a plays an important role in regulating erythropoiesis through 3 different mechanisms as shown by previous studies: EPO production, iron absorption, and hepcidin suppression.
Figure 3: Role of HIF in Anaemia and its Regulation14
Rationale for Developing HIF-PHI
HIF-PHIs stimulate erythropoiesis in a dose-dependent manner and have consistently shown clinical efficacy in phase 2 and 3 studies in both non-dialysis-dependent (NDD) and dialysis-dependent (DD) CKD anaemia patients.15 HIF degradation is prevented by the inhibition of prolyl-hydroxylases leading to increase in endogenous EPO concentration within the physiological range, rather than the pharmacological levels achieved by current ESAs. The rate of adverse events related to the high EPO levels should therefore be expected to be lower than with ESAs. Potential beneficial effects of HIF were seen in pre-clinical and early clinical studies such as an improved iron utilisation, HDL and LDL lowering effect, ischaemia protection and a protective effect on CKD progression, improved neo-vascularisation or betier blood pressure control.16 These aforesaid benefits are seen because HIF also modulates many other non-erythropoietic genes. However, tumor progression, enhanced vascular calcification, enhanced growth of renal cysts, worsening of retinopathy, or an increase in pulmonary artery pressure are the adverse effects observed due to the modulation of other genes with this new class of drugs. Moreover, it is not known if prolyl-hydroxylase inhibitors inhibit other di-oxygenases beyond HIF-PHIs. Data from large phase III trials are yet to be published and should hopefully provide some insights in this regard.15
An Introduction to Desidustat: A Peek into the Development Journey
Desidustat is a new therapeutic approach to treat anaemia secondary to chronic kidney disease (CKD). Structurally, desidustat is an oral hypoxia-inducible factor prolyl-hydroxylase inhibitor (HIF-PHI) that stimulates erythropoiesis by stabilising hypoxia-inducible factor (HIF) via prolyl-hydroxylase inhibition.
Desidustat: Physical and Chemical Properties
| Chemical Name | Glycine, N-[[1-(cyclopropylmethoxy)-1, 2-dihydro-4- hydroxy-2-oxo-3-quinolinyl]carbonyl] |
| Zydus Cadila Compound Code | ZYAN1 |
| Internatinal Nonproprietary Name | Desidustat |
| Molecular formula | C16H16N2O6 |
| Molecular Weight | 332.31 g/mol |
| Physical Form | White to off white coloured round shape uncoated biconvex tablet having plain surface on both sides |
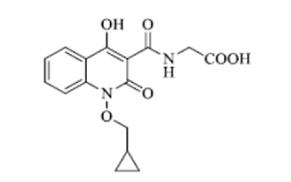
Structural Formula of Desidustat
7.2 Desidustat: Formulation
Each uncoated tablet contains:
| Desidustat | 25 mg, 50 mg |
| Excipients | q.s. |
Inactive ingredients in the tablet are microcrystalline cellulose, lactose, croscarmellose sodium, hypromellose, talc and magnesium stearate.
An in vitro sensitive liquid chromatography tandem-mass spectrometry (LC-MS/MS) assay was used to thoroughly validate ZYAN1 stability in whole blood and urine. ZYAN1 was found stable in diluted blood matrix under
benchtop for 24h, freeze-thaw cycle for four cycles, 97h for processed sample waiting for injection, freezer stability for up to 60 days at -20°C and for 336-343 days at -80 °C. (Table 1). In urine matrix, ZYAN1 stability included benchtop stability: 25h, freeze–thaw cycles: four cycles, processed sample's stability: 66h, freezer stability up to 190-191 days at -20 °C and 696 days at -80 °C. (Table 1).17
REFERENCES: