CLINICAL TRIALS
Clinical Evidences of Desidustat
Phase II studies
- The phase II trial, a randomized, double-blind, 6-week, placebo-con¬trolled study evaluated the safety, tolerability, and efficacy of desidustat in adult CKD patients (117 eligible patients) with anaemia, who were not on dialysis.19
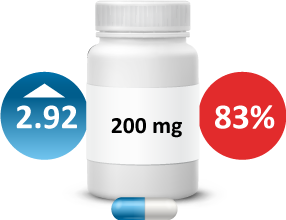
- Desidustat was administered every alternate day for 6 weeks in fasting conditions. The primary endpoint was change in hemoglobin (Hb) from baseline to Week 6. The desidustat arms (100, 150, and 200mg treatment arms) displayed a mean Hb increase of 1.57, 2.22, and 2.92g/dL, re-spectively, compared to the 0.46g/dL in the placebo arm in the modified intent-to-treat (mITT) population. Mean Hb increase of 1.70, 2.55, and 3.28g/dL was observed in 100, 150, and 200mg treatment arms, respectively, compared with 0.02g/dL in placebo arm in per protocol (PP) population. Eighteen patients had at least one treatment emergent adverse event (TEAE). No death or serious adverse event was reported during the trial.19
Is Desidustat safe and effective for anemia in CKD Patients (not on dialysis)?
Phase 2,
randomized, double-blind, 6-week, placebo-ranging, safety & efficacy study
117
eligible patients with CKD stages I-IV
Hb: 6.5-11 g/dL, Ferritin: 100-1,000 μg/L, TSAT > 20%, Body weight > 45 kg
Every alternate day for 6-weeks in fasting conditions
- Mean HB increase
- Responder rate
Secondary Endpoints19
18
patients developed at least one treatment emergent adverse event (TEAE)
NO DEATHS
or serious adverse effect
INCREASED
Desidustat, Cmax & AUC in dose-related manner.
NO CHANGE
in vital sign EKG OR safety laboratory values
Conclusion: This phase 2 study demonstrated that Desidustat is an effective and well-tolerated, oral drug for correcting anemia in patients with CKD, and provides justification for conducting phase 3 study19
References :
19. Parmar DV , Kansagra KA , Patel JC. Outcomes of Desidustat Treatment in People with Anemia and Chronic Kidney Disease: A Phase 2 Study. Am J Nephrol 2019;49(6):470-478.